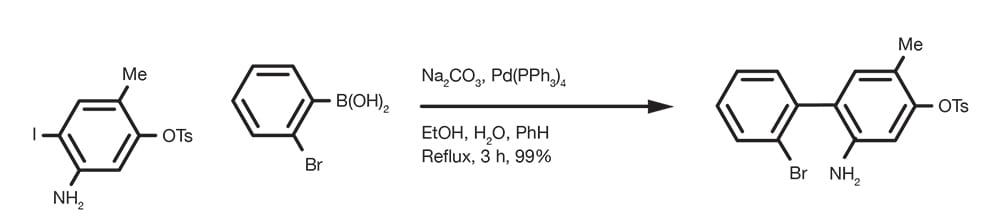
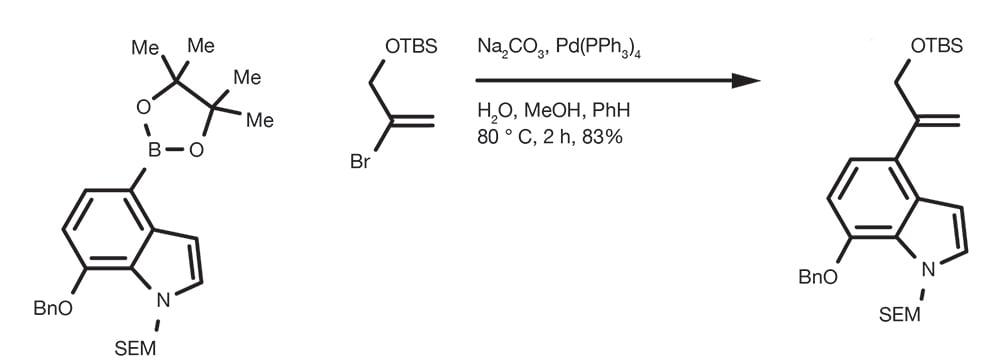
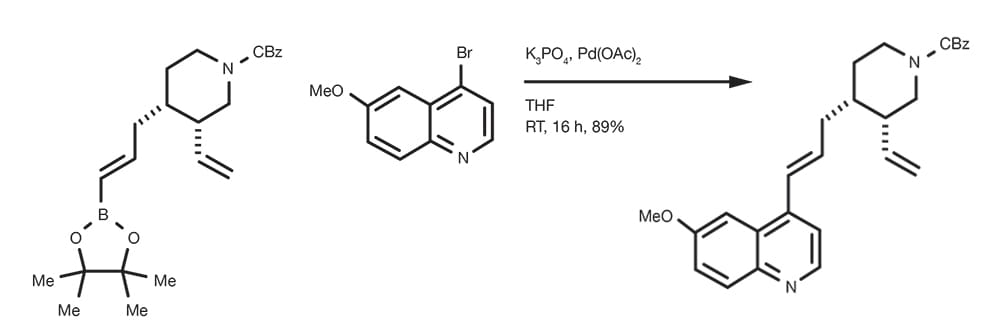
Suzuki-Miyaura Cross-Coupling Reaction
Carbon-Carbon cross-coupling reactions represent one of the biggest revolutions in organic chemistry and are currently some of the most common reactions in synthetic organic chemistry. Their invention won Akira Suzuki, Ei-Ichi Neghishi and Richard Heck the Nobel Prize for Chemistry in 2010.
Among the various type of cross-coupling, the Suzuki-Miyaura – usually simply called “Suzuki coupling” - is arguably the one with the broadest utility and applicability. The Suzuki chemistry is based on the Pd(0) catalysed coupling of an aryl or vinyl halide with an aryl or vinyl boronic acid.
Its advantages over similar reactions reside in the mild reaction conditions, common availability of the starting materials and their general low toxicity. Boronic acids are easily prepared, widely available on the market and reasonably cheap. Lower environmental impact and reduced safety hazards further add to the advantages over using organozinc or organostannane compounds. The inorganic bi-products are easily removed from the mixture. It is also often possible to run the reaction in water with obvious benefits to its green profile, while opening its scope to a wide variety of water-soluble substrates.
Since its invention in 1979, significant progresses have been made and the use of boronic acids, esters and trifluoroborates salts is widely reported; also alkyl boronic acids can be considered despite their lower reactivity (with the use of late generation catalysts).
The scope of the other coupling partner has also expanded over time to include pseudo-halides, such as triflates or aryl diazonium salts, and alkyl halides. The relative reactivity of the halide/pseudo-halide coupling partner is:
R-I > R-Br > R-OTf >> R-Cl
Aryl > Vinyl >> Alkyl
Recent generation homogeneous Pd catalysts have reduced the catalyst loading by orders of magnitude, contributing to the economy of the reaction, now utilised in numerous commercial processes. It is possible – in fact beneficial – to screen many different catalysts, from relatively simple Pd(0) complexes, such as Pd acetate and Pd tetrakis, or various forms of Pd precatalysts + phosphine ligands and fully formed (pre)catalysts, often as air-stable complexes for easier handling by the bench chemist.
Heterogeneous Pd catalysts can also be used for some simple coupling, although their reactivity is much lower than homogeneous catalysts for highly hindered substrates, or low reactivity electrophiles (e.g. Ar-Cl). The use of aryl diazonium salts, often called “super-electrophiles”, as coupling partners make heterogeneous catalysts quite an attractive option.
Reference Reaction Protocols
Weigh aryl/vinyl halide (1 mmol), and the boronic acid/ester (slight excess, 1.1 mmol), palladium catalysts (0.5-10% w/w), tetrabutylammonium bromide (1mmol) and base (2.5 mmol). Dissolve in distilled water or primary/secondary alcohol in a round bottom flask (with magnetic stirring and reflux apparatus). Heat on a sand bath to the required temperature (coupling reactions can be run from room temperature to 120-150°C). Purge nitrogen gas while stirring. Running the reaction under a nitrogen environment is recommended. Reaction times usually vary between 1-12h.
The reaction work-up can be based on filtration or extraction depending on the chemical nature of the product.
Key literature references
- Chem. Commun. 1979, 20 (36): 3437-3440.
https://doi.org/10.1016/S0040-4039(01)95429-2 - Chemical Reviews 1979, 95 (7): 2457–2483.
https://doi.org/10.1021%2Fcr00039a007 - J. Chem. Soc., Chem. Commun. 1979, 0 (1): 866-867.
http://dx.doi.org/10.1039/C39790000866 - Journal of Organometallic Chemistry 1999, 576 (1–2): 147-168.
https://doi.org/10.1016/S0022-328X(98)01055-9 - Pure and Applied Chemistry, 2009, 63 (3): 419–422.
https://doi.org/10.1351/pac199163030419 - ChemCatChem 2016, 8: 1998 – 2009
https://doi.org/10.1002/cctc.201600134
Product Selection
Catalysts/ligands:
USE THE CHEMICAL STRUCTURE SEARCH TOOL TO FIND THE CATALYST YOU NEED
Palladium(II) acetate, Pd(OAc)2 / [Pd(OAc)2]3
Palladium(0) Tetrakis, Pd(PPh3)4
Palladium(II) trifluoroacetate, Pd(CF3COO)2
Palladium(II) acetylacetonate, Pd(acac)2
and
Palladium(II) acetylacetonate, Pd(acac)2
Tris(dibenzylideneacetone)dipalladium(0), Pd2(dba)3
dichloro[1,1’-bis(di-tert-butylphosphino)ferrocene]palladium(II)
XPhos Palladacycle 2nd Gen:
Dichloro[1,1'-bis(di-tert-butylphosphino)ferrocene]palladium(II), Pd(dbpf)Cl2
Allylpalladium(II) chloride dimer, [Pd(allyl)]2Cl2
Alternative catalysts:
Bis(triphenylphosphine)palladium(II) diacetate, Pd(PPh3)2(OAc)2
Benzyl(chloro)bis(triphenylphosphine) palladium(II), Pd(PPh3)2BnCl
Dichlorobis(tri-o-tolylphosphine)palladium(II), Pd(P(o-Tol)3)2Cl2
Dichlorobis(tricyclohexylphosphine) palladium(II), Pd(PCy3)2Cl2
Dichloro(1,5-cyclooctadiene)palladium(II), Pd(cod)Cl2
Bis[1,2-bis(diphenylphosphino)ethane] palladium(0), Pd(dppe)2
Dichloro[1,2-bis(diphenylphosphino)ethane] palladium(II), Pd(dppe)Cl2
Dichloro[bis(1,3-diphenylphosphino)propane]palladium(II), Pd(dppp)Cl2
Dichloro[bis(1,4-diphenylphosphino)butane]palladium(II), Pd(dppb)Cl2
Basic Ingredients/Additives:
Silica Gel (for column purification) 40-60μm
Silica Gel (for column purification) 60-200μm
USE THE SEARCH BAR ON TOP OF THE PAGE FOR SPECIFIC SEARCHES BY PRODUCT NAME OR CAS NUMBER
Building blocks:
USE THE CHEMICAL STRUCTURE SEARCH TOOL TO FIND THE BUILDING BLOCK YOU NEED
(het)aryl halides:
Ethyl 4-chloro-6-methoxyquinoline-3-carboxylate
(het)aryl OTf:
2-Naphthyl trifluoromethanesulfonate, 97%
4-Nitrophenyl trifluoromethanesulfonate, 99%
Vinyl OTf:
3,6-Dihydro-2H-thiopyran-4-yl trifluoromethanesulfonate
(het)aryl boronic acids
2-Bromobenzeneboronic acid, 98%
Vinyl trifluoroborates:
Potassium 4-methyl-beta-styryltrifluoroborate, 95%
Potassium vinyltrifluoroborate, 97%
Aryl diazonium salts:
4-Nitrobenzenediazonium tetrafluoroborate, 97%