Amine Protection / Deprotection
Boc-Protection / Deprotection
1-PROTECTION
The BOC (tert-butyloxycarbonyl) protecting group, chemically a di-tert-butyl dicarbonate (Boc2O), is probably the most common amine protecting group in non-peptide chemistry. The reaction conditions for the amine protection are quite flexible. The process usually achieves high yields and fast conversions under relatively mild conditions.
It is typically performed in water, water/THF, THF, or acetonitrile at room temperature or moderate heat (40°C) in the presence of a base. Dioxane and methanol have also been reported as solvents for the reaction, while an alternative common method is refluxing a biphasic mixture of chloroform and water, with sodium bicarbonate as a base.
Common bases for the transformation are sodium hydroxide, 4-dimethylaminopyridine (DMAP) and sodium bicarbonate.
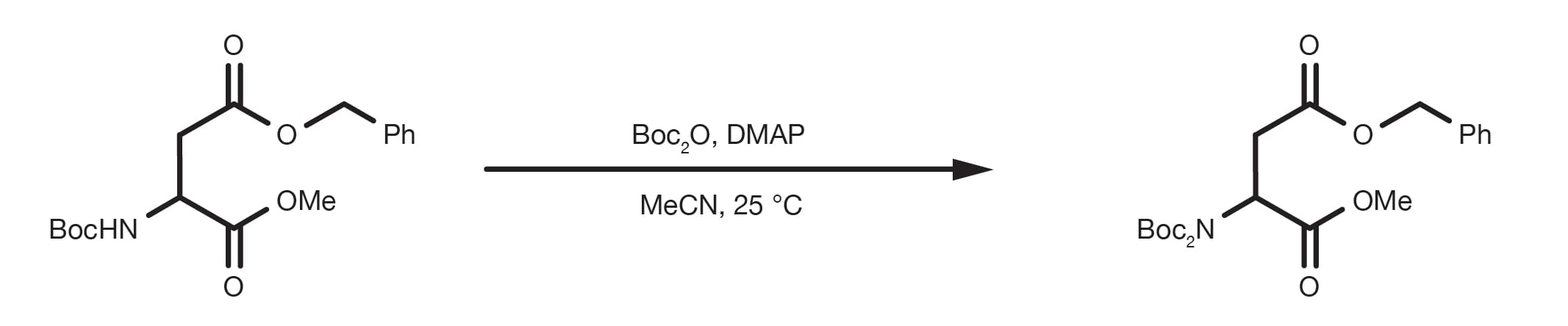
A completely different route for the preparation of protected amines is the direct reaction of an alkyl halide with di-tert-butyl-iminodicarboxylate. Even though this is not strictly an amine protection, the method can be useful in the design of the synthetic strategy.
Reference Reaction Protocols
Prepare a solution in the solvent of choice containing the amine, 2-3 equivalents of BOC, and 1-1.5 equivalents of base. Stir at room temperature or moderate heat (40°C). Water dilution and a subsequent extraction are typically used to isolate the product.
Examples
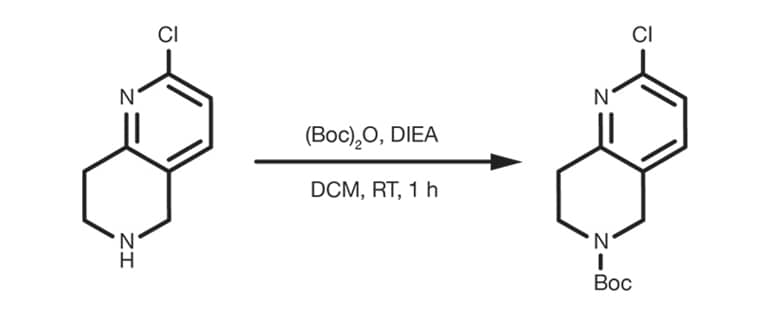
Patent Reference: WO2012129344
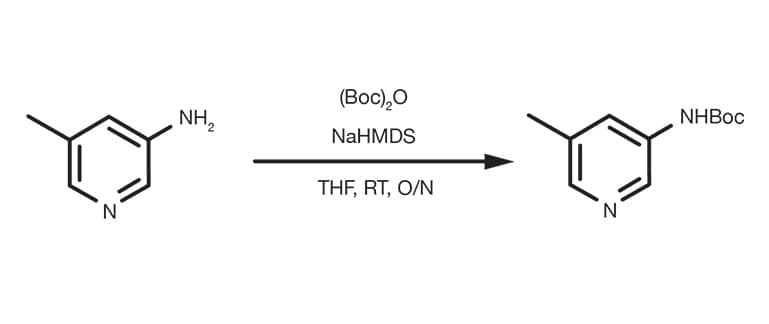
Patent Reference: WO2010026121
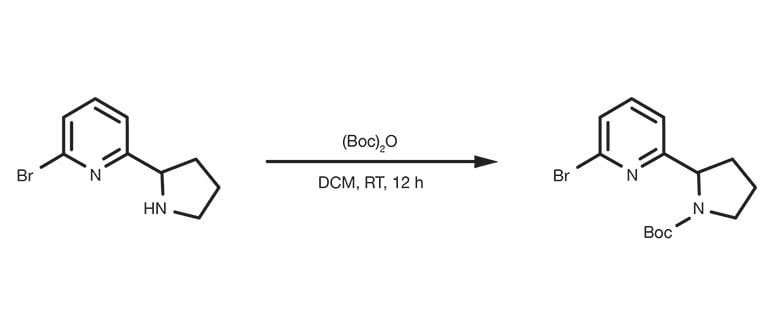
Patent Reference: WO2016011390
2-DEPROTECTION
The deprotection of a BOC-protected amine is a simple carbamate hydrolysis in acidic conditions.
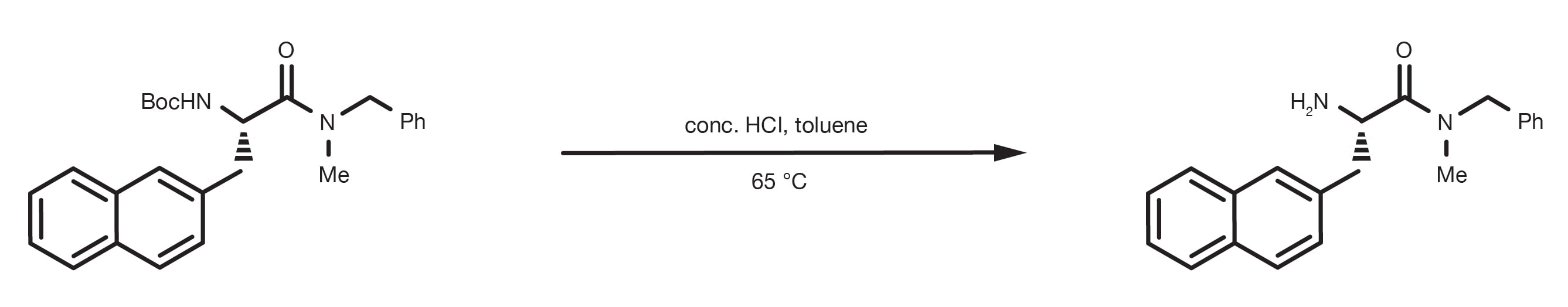
The starting material is dissolved in water or organic solvent, such as toluene, dichloromethane, or ethyl acetate. Concentrated hydrochloric acid, or trifluoroacetic acid (TFA) are the acids of choice. The reaction is usually fast and happens at room temperature. Biphasic systems can be used, with the protected amine dissolved in the organic phase mixed with the aqueous solution of the acid.
Two other common deprotection methods avoid the use of a strong acid. They can be useful for acid-labile compounds. The trade off is a slightly more complex reaction set up and longer reaction times, up to 48-72h.
The two reagents that can be used are trimethylsilyl iodide (TMSI) and zinc bromide. The starting material is dissolved in an organic solvent, such as dichloromethane, together with the reagent (the TMSI is usually added dropwise) and the reaction is run under stirring at room temperature for 12-24h.
Reference Reaction Protocols
Acid hydrolysis
The protected amine is suspended in a 4M HCl aqueous solution, stirred at room temperature for 2h and isolated under vacuum.
Alternatively, the protected amine dissolved in DCM is treated with concentrated TFA, or a 25% TFA/DCM solution, stirred at room temperature for 2-12h. Vacuum evaporation of the solvent is sufficient to isolate the product.
Alternative method
Dissolve the protected amine in DCM, add an excess of zinc bromide (2-3 equivalents), or TMSI (1.2-1.5 equivalents added dropwise), stir at room temperature overnight. Isolate the product by removing the solvent under vacuum.
Examples
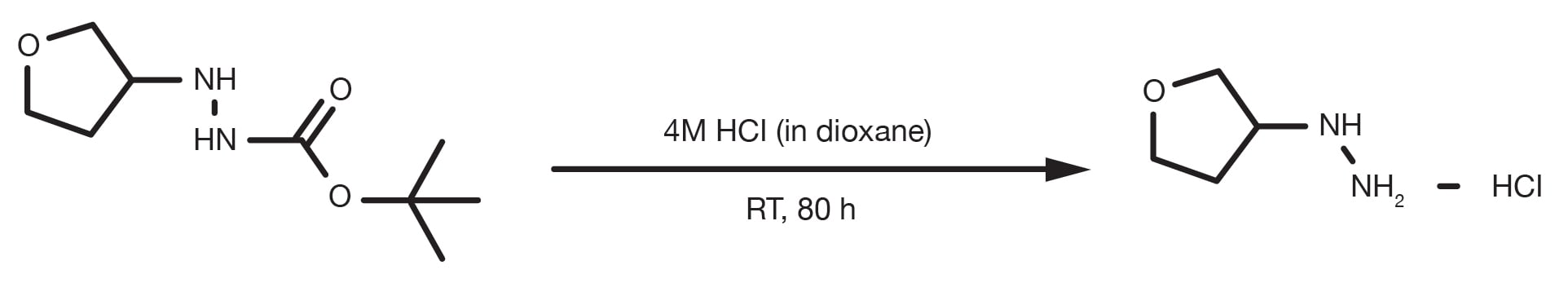
Patent Reference: WO2010032200
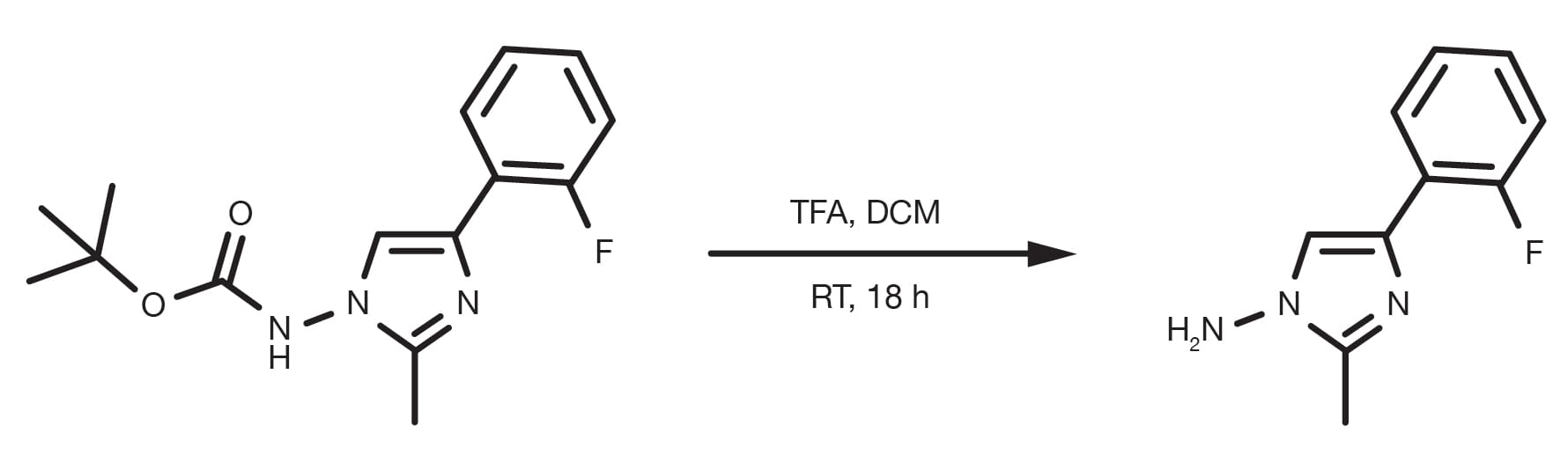
Patent Reference: WO2014177977
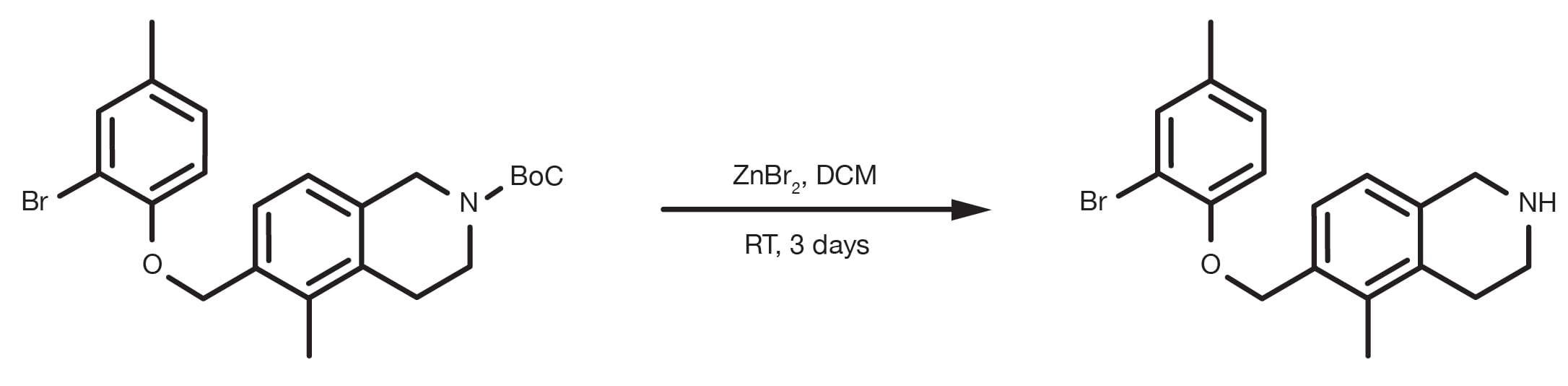
Patent Reference: WO2016014463
Key literature references
- Wuts, Peter G. M.; Greene, Theodora W. "Protection for the Amino Group". Greene's Protective Groups in Organic Synthesis (4th ed.). John Wiley & Sons. pp. 696–926. doi:10.1002/9780470053485.ch7
- Rules for Abbreviation of Protecting Groups, Pure Appl. Chem. 85, 307 (2013). DOI: https://doi.org/10.1351/PAC-REP-12-07-12
Product Selection
PROTECTION
Basic ingredients / Additives:
Sodium Bis(trimethylsilyl)amide (NAHMDS) solution
Sodium Bis(trimethylsilyl)amide (NAHMDS) solid
Reaction work-up:
Silica Gel (for chromatographic purification)
USE THE SEARCH BAR ON TOP OF THE PAGE FOR SPECIFIC SEARCHES BY PRODUCT NAME OR CAS NUMBER
DEPROTECTION
Basic ingredients / Additives:
Reaction work-up:
Silica Gel (for chromatographic purification)
USE THE SEARCH BAR ON TOP OF THE PAGE FOR SPECIFIC SEARCHES BY PRODUCT NAME OR CAS NUMBER
Benzylation / Debenzylation
1-PROTECTION
The Benzyl (Bn) derivative is another popular amine protecting group. The N-benzylation happens by reaction of the amine with benzyl halide in the presence of a base.
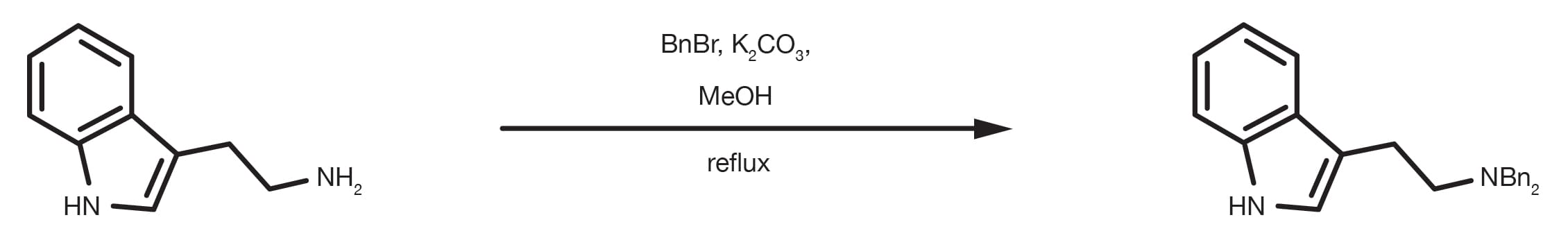
Common solvents for the reaction are methanol, other primary alcohols, DMF, acetonitrile, and some other aprotic polar organic solvents.
See “Amination of alkyl halides” section for more details on the chemistry and reference reaction protocols.
An alternative, less common, method to introduce the protecting group on the amine is the reductive amination of benzaldehyde with sodium cyanoborohydride (NaBH3CN) in acidic conditions (HCl 6M), typically performed in methanol.
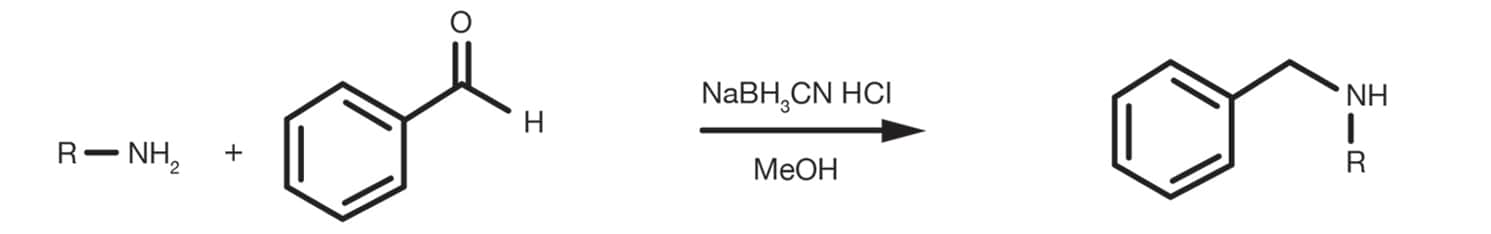
Reference Reaction Protocols
Prepare a solution of the amine in methanol or acetonitrile, add 3 equivalents of benzyl bromide and 3 equivalents of base (typically potassium carbonate). Stir the reaction at reflux for 3-6h. Upon completion, quench the reaction mixture with water and extract the product with an appropriate organic solvent (e.g. ethyl acetate).
Reductive amination:
Prepare a solution of hydrochloric acid 6M in methanol adding the amine and 5 equivalents of benzaldehyde. Stir the mixture and cool it to 0C, then add sodium cyanoborohydride (1.5 equivalents). Stir the reaction at room temperature for 6-12h. Isolate the product by solvent evaporation under vacuum and proceed to the purification.
Key literature references
- Czernecki, S.; Georgoulis, C.; Provelenghiou, C. Tetrahedron Lett. 1976, 17, 3535. doi:10.1016/S0040-4039(00)71351-7
- White, J. D.; Reddy, G. N.; Spessard, G. O. J. Am. Chem. Soc. 1988, 110, 1624. DOI: 10.1021/ja00213a047
Examples

Patent Reference: WO2014149164
2-DEPROTECTION
The Bn protecting group is selectively cleaved by Pd-catalysed hydrogenolysis.
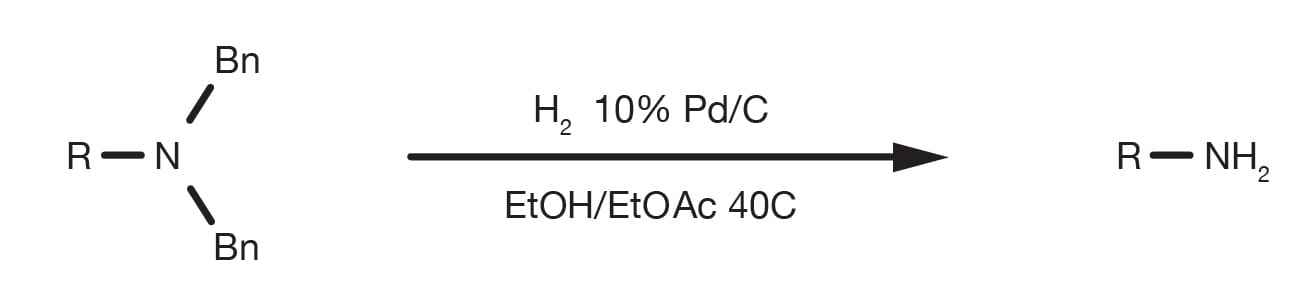
The reaction can be performed in numerous solvents under 1-3 bar H2 and moderate heat. The catalyst of choice is 10% Pd/C. As there is no need for high hydrogen pressure, standard laboratory glassware can be used. Dedicated apparatus, such as a Parr shaker hydrogenator would certainly help, while only in certain specific situations, a dedicated autoclave is necessary.
A key element for the success of the deprotection is the choice of the right catalyst. Several different Pd/C variants are commercially available and their relative performances differ quite dramatically depending on the nature of the starting material and the reaction conditions. A 10% Pd/C with eggshell deposition is the catalyst of choice for this deprotection. Screening multiple catalysts is often necessary to identify optimal conditions for the reaction.
The handling of heterogenous Pd catalysts requires some care, as a dry catalyst can be pyrophoric, even though under normal “wet” conditions and during the reaction it is regarded as a safe and very scalable chemistry.
Reference Reaction Protocols
Prepare a solution of the benzylated amine in ethanol in a Parr flask. Purge with N2 then add 30% w/w catalyst (10% Pd/C). Run the hydrogenation in the Parr apparatus under 40 psi H2 for 1-3h. At the end of the conversion filter the catalyst and recover the product by solvent evaporation. Proceed to purification.
Examples
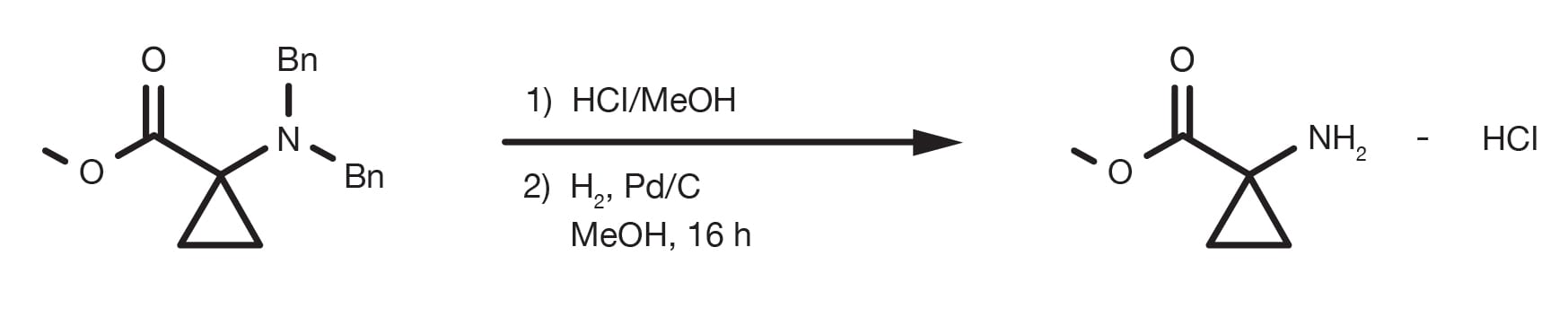
Patent Reference: WO2014149164

Patent Reference: WO2016014463
Key literature references
- Martin Studer, et al.; Influence of Catalyst Type, Solvent, Acid and Base on the Selectivity and Rate in the Catalytic Debenzylation of 4-chloro-N,N-dibenzyl Aniline with Pd/C and H2; Journal of Molecular Catalysis A: Chemical 112 (1996) 437-445.
- Self, Louis S., et al. "Selective Hydrogenolysis of Benzyl and Carbonbenzyloxy Protecting Groups for Hydroxyl and Amino Functions," Catalysts of Organic Reactions, 1990 147-716.
Product Selection
PROTECTION
Solvents:
SEE ALL OUR SOLVENTS - OVER 1000 ALTERNATIVES
Extraction often done in hydrophobic solvents:
Basic ingredients / Additives:
Sodium cyanoborohydride, NaBH3CN
USE THE SEARCH BAR ON TOP OF THE PAGE FOR SPECIFIC SEARCHES BY PRODUCT NAME OR CAS NUMBER
DEPROTECTION
Basic ingredients / Additives:
USE THE SEARCH BAR ON TOP OF THE PAGE FOR SPECIFIC SEARCHES BY PRODUCT NAME OR CAS NUMBER

