Large-Scale Production of Lentiviral Vectors by Transient Transfection
By Matthew Weston
What are Lentiviral Vectors
Lentiviral vectors (LVs) are used for gene transfer in cell and gene therapy applications.[1] They have been demonstrated to be safe and effective for use in clinical treatments and, as of 2022, were employed in more than 100 ongoing clinical trials around the world for ex vivo modification of cells or in vivo therapy.[2] Indeed, as a result of this popularity and the recent approval of certain CAR-T therapies, the market for LV production is predicted to grow to $800 million by 2026.[3] Notable treatment areas include hereditary diseases such as ß-thalassemia[4] or Parkinson’s disease.[5]
"As a result of this clinical trial popularity and the recent approval of certain CAR-T therapies, the market for LV production is predicted to grow to $800 million by 2026."
The Need for Scale-Up
As the use of LVs in new therapies has increased, so has the need for technologies that can help increase their manufacture. The large-scale production of LVs is currently generally achieved by directly amplifying small-scale production methods, for example by expanding the cultivation surface area by addition of supplementary cultivation/production units (or ‘scaling-out’). There is thus considerable interest in determining the best equipment set-up for optimal production, and XIAOFAN AND RUI have studied LV production using the Corning™ HYPERStack™ Cell Culture Vessel,[1] which features Corning’s proprietary High Yield PERformance (HYPER) technology. In their report three Corning™ vessels are used for comparison purposes: a T-25 CellBIND™ surface flask, a 2-layer CellBIND™ CellSTACK™ culture chamber, and a HYPERStack™ 12-layer cell culture vessel.
"The large-scale production of LVs is currently generally achieved by directly amplifying small-scale production methods, for example by expanding the cultivation surface area by addition of supplementary cultivation/production units (or ‘scaling-out’)."
Comparison of Cell Culture Vessels
293T cells (ATCC CRL-3216) were cultured in Dulbecco’s Modified Eagle's Medium (DMEM) with Fetal Bovine Serum (FBS) and an antibiotic/antimycotic, seeded into the comparison flasks, and grown overnight before transfection. A three-plasmid DNA system was prepared and transfection conditions were optimized using mathematical methods.[6] The transfection complex mix was prepared and allowed to stand for a short time before fresh medium was added. Old media in the comparison vessels was discarded, the transfection mixture was added, and the vessels were incubated under controlled conditions. Supernatants were harvested and fresh media added at 48h and then at 72h, and the extracts were combined. Growing 293T cell populations were seeded onto 12-well plates, incubated, and LV-containing supernatant was added. Following further incubation, LV titres were quantified by fluorescence-activated cell sorting analysis (Figure 1).
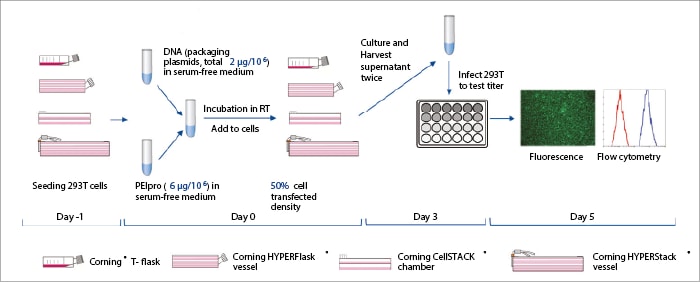
After production and titration, the HYPERStack™ 12-layer vessel achieved yields of 2.9 x 107 TU/mL and 1.2 x 107 TU/cm2, in which the total titre reached 7.5 x 1010 TU. The unit volumes and area yields of the 2-layer CellSTACK™ and T-25 flasks were lower (Figure 2).
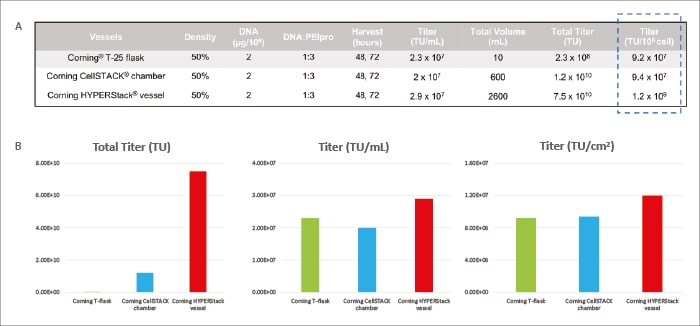
"The Corning™ HYPERStack™ vessel was compared with traditional culture vessels, revealing that the HYPERStack™ vessel can produce a superior LV titer."
Advantages of Corning™ HYPERStack™
The Corning™ HYPERStack™ cell culture vessel combines the best of two products - the Corning™ CellSTACK™ and HYPERFlask™ cell culture vessels.[7] HYPER technology features a unique, ultra-thin gas-permeable film to eliminate the air gap within the vessel. This results in a substantial increase in cell growth surface area compared to traditional stacked-layer vessels of comparable cubic footprint. The utilization of HYPER technology in the spatial footprint of the CellSTACK™ delivers an efficient, scalable cell culture vessel for adherent cell culture.
From viral vector vaccine production to stem cell therapy, Corning™ HYPERStack™ vessels offer many benefits, including:
- Higher total cell yields - up to 5x the growth surface area of a traditional cell culture vessel of comparable footprint
- Similar cell morphology, phenotype, and growth compared to traditional vessels
- Closed system design ideal for large scale cells, protein therapeutics, stem cell therapy, extracellular vesicles, vaccines, and virus production, including those targeting COVID-19
- Scalable product with multiple size offerings to support scale-up and scale-out
- Innovative assembly and ergonomic design
- Less volumetric waste - offers a fixed media volume of 0.2 mL/cm2 fills
Matthew Weston is a Content Specialist at Fisher Scientific UK.
References
[1] Xiaofan T, Rui C. Large Scale Transient Transfection for the Production of Lentiviral Vectors using the Corning™ HYPERStack™ Cell Culture Vessel: Application Note. Corning Inc. 2022. www.corning.com/catalog/cls/documents/application-notes/CLS-AN-669.pdf.
[2] Milone MC, O’Doherty U. Clinical Use of Lentiviral Vectors. Leukemia 2018; 32: 1529–1541. Doi: 10.1038/s41375-018-0106-0.
[3] Southey F. Oxford BioMedica to Capture 25-30% of Lentiviral Vector Market by 2026, Predicts CTO. Dec 10, 2018. Retrieved from www.biopharma-reporter.com/Article/2018/12/10/Oxford-BioMedicato-capture-25-30-of-lentiviral-vector-market-by-2026-predicts-CTO.
[4] Cavazzana-Calvo M, et al. Transfusion independence and HMGA2 activation after gene therapy of human ß-thalassaemia. Nature 2010; 467: 318–322. Doi: 10.1038/nature09328.
[5] Palfi S, et al. Long-Term Safety and Tolerability of ProSavin, a Lentiviral Vector-Based Gene Therapy for Parkinson’s Disease: A Dose Escalation, Open-Label, Phase 1/2 Trial. Lancet 2014; 383: 1138–1146. Doi: 10.1016/S0140-6736(13)61939-X.
[6] Yuan W, et al. Comparative Analysis and Optimization of Protocols for Producing Recombinant Lentivirus Carrying the anti-Her2 Chimeric Antigen Receptor Gene. J Gene Med 2018; 20(7–8): e3027. Doi: 10.1002/jgm.3027.
[7] Corning™ HYPERStack™ Cell Culture Vessels: Multiply Your Cells, Not Your Work. Corning Inc. 2023. www.corning.com/emea/en/products/life-sciences/products/bioprocess/hyper-platforms/hyperstack-platform.html.